Comparison of Electronic Batch Recording Systems in the pharmaceutical industry
Part 1
IT
Key Words Electronic batch recording solution | paperless manufacturing | MES
Abstract
During the last years the technology around Electronic Batch Recording Systems (EBRS) has made huge progress. Despite many examples of successful EBRS implementations traditional paper batch tickets remain common practice in the pharmaceutical industry. The benefits of EBRS deployment to compliance, cycle time, efficiency and costs are in conflict with massive change management efforts and investments required for system deployment.
Until now, the cost-benefit evaluation for these solutions is difficult. Existing evaluation modes are inadequate since they (i) primarily rely on using investment costs, (ii) neglect intangible performance measures, (iii) leave out performance deteriorations, (iv) are not available for different EBRS implementation designs and (v) do not consider the effects from structural, process technological and organizational differences of the company.
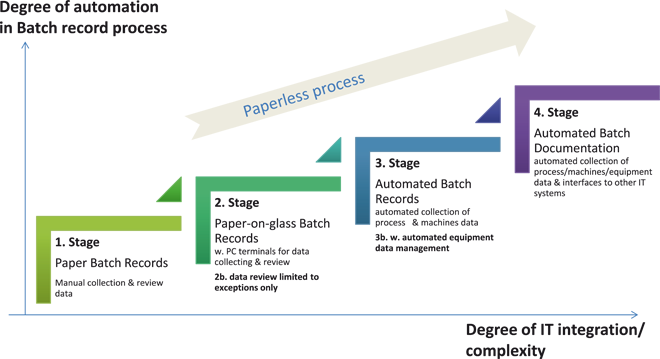
The study compares the benefits and weaknesses for selected generic EBRS/MES (Manufacturing Execution System) solutions types. The results are the outcome of a survey with several experts from ten different multi-sites pharmaceutical companies and organizations. The participants of the survey were asked to assess five different EBRS/MES solutions types on a set of evaluation criteria. Each of the selected EBRS/MES solutions type differs in its vertical and horizontal integration depths of the EBRS/MES with other information technology systems and process automation and in the automation degree of the batch documentation process.
The study supports decision makers in finding the most effective batch recording solution optimizing their operations and supply chain performance taking both, specific technical integration needs and organizational structures, into account. The reader gains insights into possible improvements in costs, flexibility, quality, information transparency and speed through EBRS deployment. Furthermore, important take-aways for a successful implementation as well as estimated investment efforts are presented.
Correspondence:
Monika Futschik, Roche Diagnostics GmbH, MMBFML..6164, Sandhofer Strasse 116, 68305 Mannheim;
e-mail: monika.futschik@roche.com
 | Monika Futschik Monika Futschik holds Master degrees in Mechanical Engineering and in Supply Chain and Operations Management from the University of Liverpool. After working in the automotive industry she joined Roche Diagnostics in 2006 where she is now responsible for the department Finished Goods & Operations Support within the sterile drug product manufacturing in Mannheim, Germany. In parallel to her job, she is completing her PhD at the University of Duisburg-Essen about |
Schließen Sie hier ein Abonnement ab und profitieren Sie von den vielseitigen Nutzungsmöglichkeiten.