An overview of sterility testing
The impact of Annex 1 and a look into the future of sterility testing
Produktion
Abstract
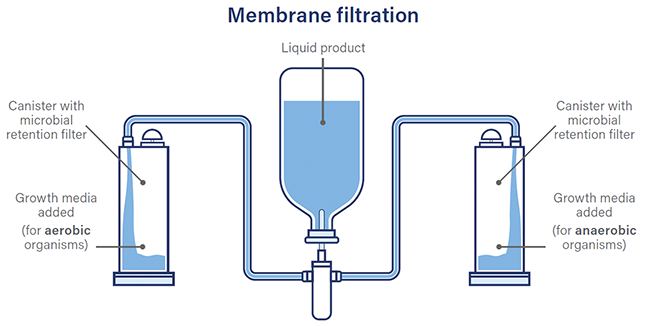
Sterility testing is a crucial process in the manufacture of sterile products, ensuring they are free from harmful microorganisms. Two primary methods are used: membrane filtration and direct inoculation, each with challenges such as long wait times and the risk of false positives. Insourcing versus outsourcing sterility testing presents various advantages and disadvantages. The industry is increasingly adopting isolators to minimize contamination risks, supported by regulatory guidelines like EU GMP Annex 1. Isolators offer benefits in reducing false positives and operating costs. The future of sterility testing focuses on developing rapid testing methods to enhance efficiency and reduce wait times, especially vital for products with short shelf lives, such as cell therapies.
Korrespondenz:
Chris Berridge, Ecolab Ltd., 52 Royce Cl, Andover SP10 3TS, UK; Berridge, christopher.berridge@ecolab.com
 | Chris Berridge is a seasoned Biomedical Science BSc (Hons) graduate with over 9 years of expertise in hydrogen peroxide vapor bio-decontamination within GMP-grade cleanrooms. He excels in bio-decontamination services and equipment validation, ensuring top-tier cleanliness and safety. With 4 years of project management experience, he specializes in the installation and integration of bio-decontamination systems for rooms and enclosures. As an IRCA Certified GMP Associate Auditor, he is dedicated to maintaining |
Schließen Sie hier ein Abonnement ab und profitieren Sie von den vielseitigen Nutzungsmöglichkeiten.